Biopharma Project Introduction
I.Project Overview
This project is a Real World Asset (hereinafter referred to as the “RWA Project”) type project, and the BVI company----Fortune Well Corporate Limited, BVI company number is 2178072 (hereinafter referred to as the “Issuer”) will tokenize all its equity. The total minting scale of tokens will be locked at 50 million, the Token Name is “Biopharma”, the Token Symbol is “BIOPH”
The issuer owns the full and complete income rights of 19.14% equity of a target company through legal agreements (hereinafter referred to as “Target Company” or “Underlying Asset”).Including but not limited to profit distribution rights, dividend distribution rights , equity transfer income and other types of income rights , but excluding voting rights). The specific structure is shown in the figure below:
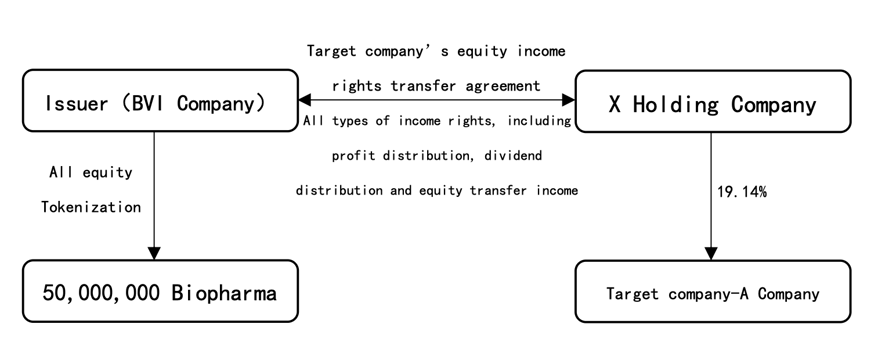
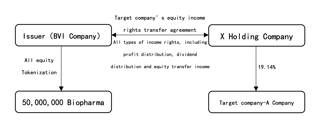
II. Overview of the Target Company
The target company is a biopharmaceutical technology R&D company that has been established in China for ten years and has an innovative drug R&D platform as its core competitiveness. It is committed to becoming a pioneer in the integrated drug R&D system , and has the feasibility of optimizing and upgrading the vast majority of marketed drugs; it has reserved multiple new molecular entities(New molecular entity : Active Pharmaceutical ingredient that has never been approved or marketed as drug;) and a class of innovative drugs with independent intellectual property rights; it will continue to optimize and integrate the latest technologies, continuously extend the scope of application , and strive to create high-quality, affordable innovative drugs for patients.
III. R&D, and innovations of the target company
Over the past decade, the target company has invested a lot of resources in R&D and achieved remarkable results:
1. AI-NANO Innovative Drug R&D platform: The innovative drug R&D platform called AI-NANO developed by the target company combines the " AI-driven Chemical Drug Screening System" and the " Nano-Delivery System Improving the Pharmacokinetics, NDSIP drug delivery system", a game changer compares to the traditional R&D model.
Its AI-driven chemical drug screening system takes marketed drugs as the starting point and modifies their pro-drug structures . It also simultaneously screens and matches the drug delivery systems while performing structural modifications. It studies the efficacy, pharmacokinetics and drug delivery systems in the same system.
The NDSIP drug delivery system uses nanoparticle formulations release the active drug after entering the circulatory system through the gastrointestinal tract, which greatly improves the oral bioavailability of the drug. The tissue distribution of the drug in the body has better targeting specificity. And it’s more suitable for poorly water-soluble drugs.
Theoretically, the vast majority most of the drugs in the world today can be improved using the target company's AI-NANO innovative drug R&D platform, and have substantial advantages of significantly fewer adverse reactions, shorter R&D cycle, lower cost, and higher success rate. The success rate is 2.5-3 times that of the traditional model.
2. BTK inhibitor, one of the blockbuster innovative drugs under development: BTK inhibitor is currently the most important class of drugs for the treatment of non-Hodgkin lymphoma (NHL) and chronic lymphocytic leukemia (CLL) . Ibrutinib is the first irreversible covalent BTK inhibitor to be marketed and has long been one of the top 20 best-selling drugs in the world , with a global annual sales volume of nearly US$10 billion.
However, the existing ibrutinib drugs on the market have the following clinical deficiencies: 1) Adverse reactions: ≥ Grade 3, adverse events include infection, hypertension, atrial fibrillation and major bleeding. The risk of bleeding after oral administration of ibrutinib is as high as 66% , and a small number of patients, 1-8%, have a risk of severe bleeding. 2) Liver damage: Heavy intestinal and hepatic first-pass metabolism and low bioavailability predispose to drug-related liver damage. 3) Mutation resistance: About 19% mutation resistance (C481S) in 4 years . 4) Poor tolerability: About 40% of patients choose to stop taking the drug because of drug intolerance.
Compared with BTK inhibitor screened and developed by the target company's AI-NANO innovative drug R&D platform, it has the following significant advantages: 1) Better absorption: The bioavailability is greatly improved compared with ibrutinib, reducing the burden of first-pass metabolism on the liver. 2) Good pharmacodynamic properties: Lower effective dosage and better efficacy at the same dosage. 3) Better tolerability: Higher dosage can be tolerated with good efficacy and tolerability. 4) Good pharmacokinetic properties: More enriched in focal tissue sites, lower circulatory drug exposure, fewer off-target adverse effects, and substantially reduced incidence of adverse reactions. 5) Better binding inhibition activity for mutant BTK C481S than for non-mutant BTK: It can resist C481S mutation resistance to a certain extent. 6) More potential clinical value : In the face of indications that BTK inhibitor have not yet conquered (such as DLBCL, etc.), there is an opportunity to become a challenger in a new field. It is very likely to apply for rapid approval and listing as an orphan drug (treatment of a subtype of relapsed and refractory DLBCL) .
3. TNF inhibitor, the second one of blockbuster innovative drugs under development: Lenalidomide, a reference drug for TNF inhibitor developed by the target company through the AI-NANO innovative drug R&D platform, is the most effective drug for multiple myeloma so far, with annual sales exceeding US$10 billion for many years. The fluctuation of blood drug concentration of TNF inhibitor is slowed down, and the peak blood drug concentration is reduced, which effectively solves the adverse reactions caused by the large fluctuation of blood drug concentration of lenalidomide. Its NDSIP Oral bioavailability is several times that of the prototype drug lenalidomide. It has stronger targeting, lower side effects and better clinical effectiveness.
4. Other new drugs under development in the pipeline.The target company has also reserved innovative drugs with great market competitiveness in three major disease areas: tumors, autoimmune diseases, and anti-infection.
IV. Future Development of the target company
After ten years of dedicated development, the target company has built a breakthrough AI-NANO Innovative Drug R&D platform, and has developed and screened out blockbuster innovative drugs with great competitive advantages and huge market potential, such as BTK inhibitor and TNF inhibitor, and has deployed more than 20 core patents worldwide. The company was valuated for more than US$700 million by Frost & Sullivan in 2023 , and its current valuation is expected to exceed US$3 billion. The target company is already in the process of listing and is expected to be listed on the Nasdaq in 2025. Therefore, the specific name of the target company will not be disclosed and it will be announced until the listing is completed. The target company's core blockbuster new drugs under development are significantly superior to the existing main drugs on the market in terms of tolerability, efficacy, adverse reactions, etc., and have very large potential clinical value. Whether it is licensing-out or commercial development, there is huge value growth and release space in the later stage.
Looking ahead, the target company will continue to increase its investment in scientific research and continuously advance its proprietary R&D platform to develop best-in-class innovative drugs. It will continue to develop clinical and preclinical product pipelines with innovative mechanisms of action and move towards the development of new drugs. The target company will seize global market opportunities and explore various cooperation models to maximize the global value of innovative drugs .
According to statistics, in the first quarter of 2025, Chinese innovative Biopharma and Biotech companies’BD transactions soared to US$36.9 billion. Among them, 3SBio and Pfizer's single down payment of up to US$1.25 billion and the total order amount of US$6 billion for the dual-antibodylicense-outset a new high for the amount of Chinese innovative Biopharma and Biotech companies’ license-out deal. In addition, The United Laboratories (UBT251 waslicensed-outto Novo Nordisk, with a down payment of US$200 million) and Hengrui Medicine (HRS-5346 waslicensed-outto cooperate with Merck, with a down payment of US$200 million). At the ASCO 2025 annual meetingthat just ended not long ago, the data of Chinese innovative Biopharma and Biotech companies showed that Chinese innovative Biopharma and Biotech companies have strong competitiveness in R&D. The record-breaking order scale and high-frequency BD transactions of Chinese innovative Biopharma and Biotech companies are also the most direct proof that the strength of Chinese innovative Biopharma and Biotech companies has been recognized by multinational pharmaceutical companies. This has also directly driven the sharp rise in the share prices of Chinese innovative Biopharma and Biotech companies in the US and Hong Kong stock marketsin the past several months. Chinese innovative Biopharma and Biotech companies with market values of tens of billions of US dollars and hundreds of billions of Hong Kong dollars have emerged one after another, and the value of Chinese innovative Biopharma and Biotech companies is being systematically re-evaluated by the global capital market. It is expected that the market value of the target company will have huge potential for growth after listing.
V. Funding usage and future development of this project
The issuer will focus on the Biotech and BioPharma industries. The funds raised from this project will be used to continuously search for high-quality companies with great value and development potential similar to the target company, invest in a manner similar to this project, and promote the listing of investment targets to achieve sustained value growth.
After obtaining profit distribution, dividend distribution, equity transfer income and other income from the underlying assets, the issuer will public a distribution announcement within 5 working days, and will distribute all the income obtained equally to the wallet accounts of all token holders as of the deadline announced in the distribution announcement by means of AirDrop within 10 working days after the announcement.